
In Summaryīoth long and short wavelength UV light are damaging to DNA, but in different ways. However, errors in repair commonly lead to the replacement of cytosine for thymine, thus changing the original DNA sequence in potentially detrimental ways. The structural change brought about by dimerizing mutations leaves the plasmid DNA available to repair enzymes.
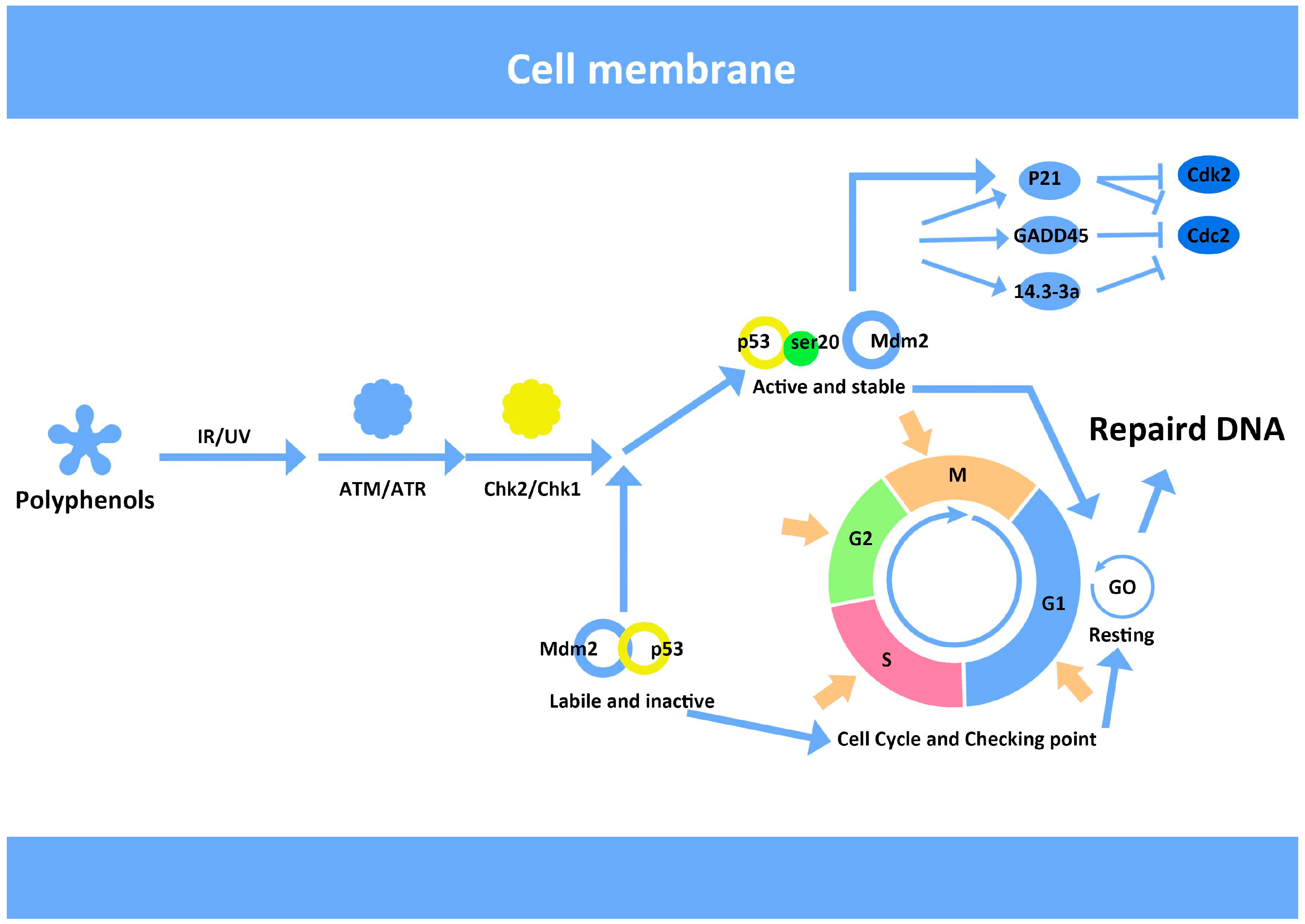
Well, a plasmid that contains UV-induced dimerizing mutations is unlikely to be replicated efficiently in E. Images by Lynnea Waters, constructed with eMolecules®. When the second strand is synthesized, the base position originally occupied by a guanine is then replaced with a thymine, leading to a G to T transversion. Instead, during replication, 8-oxoguanine can base pair with adenine via two hydrogen bonds. The oxidation of guanine into 8-oxoguanine prevents the hydrogen bonding required to base pair with cytosine. One example of this is a G to T transversion mediated by reactive oxygen species. These oxidized bases don’t pair correctly during replication, causing mutations.
#Uv photo activation repair free#
In fact, UV-A radiation commonly causes the creation of a free radical that then interacts with and oxidizes DNA bases. UV exposure doesn’t always lead directly to mutations in the DNA. This photochemical product causes a structural kink in the DNA that prevents the pyrimidines from base pairing, and prevents DNA replication. These four carbons form a cyclic ring (cyclobutane) that links the two pyrimidines, thus creating a chemical intermediate that is not normally found in DNA.
This product forms when two adjacent pyrimidines (thymines, TT, or cytosines, CC) become linked covalently by their C=C double bonds. The most common photochemical product in DNA is a cyclobutane pyrimidine dimer. UV damage occurs via two distinct types of mutations: The shorter wavelength UV-C light carries with it a lot more energy than its long wave UV-A counterpart, and is much more damaging to DNA. UV-B and UV-C rays are two types of high-energy radiation that are capable of ionizing (removing electrons from) molecules in a process called a photochemical reaction that leads to new molecular products. This range is further divided into short wave (200-280 nm, UV-C), middle wave (280-315 nm, UV-B), and long wave (315-400 nm, UV-A) light. Ultraviolet (UV) light is the part of the electromagnetic spectrum between 200 and 400 nm, with shorter wavelengths than violet of the visible spectrum (hence the name, ultraviolet). In this article, we outline what you need to know about UV light and two mechanisms by which it can cause mutations: dimerizing mutations and oxidative mutations. In cells, DNA repair mechanisms can fix UV-damaged bases, but in purified plasmids, no such mechanisms exist, and unrepaired UV damage can be detrimental to the success of downstream applications.
#Uv photo activation repair skin#
Understanding how UV causes mutations is important in understanding the mechanisms underlying skin cancer, but also in understanding the detrimental effects that UV light can have on our DNA-based experiments. UV-A and UV-B rays from the sun interact with the DNA in our skin and can cause mutations that ultimately may lead to cancer.

We all know that we are supposed to put on sunscreen in the summer months to protect ourselves from skin cancer, and the connection between sun exposure and cancer is well documented (Koh et al., 1996 Armstrong and Cust, 2017).


 0 kommentar(er)
0 kommentar(er)
